* I'll be updating this post a little more later. *
I'd thought I'd share my new method of germinating seeds. Last year I had some mixed results germinating seeds using various methods like the wet paper towel method and popping beans directly into peat and rockwool. Most of the time I would close to if not 100% success but the odd time I would be down in the 40% range. If you're like me and like to spend a bit of your hard earned cash on beans you don't want them to fail as they cost a lot! For this reason, I ran several experiments using a whole bunch of old seed and some seeds that were from a pollen chucking project a couple of years ago and see what method would work the best as well as be the most consistent. After burning through about 100 seeds, the method outlined below was not only the fastest by far, it was the most reliable with a 100% success rate.
With any method, there's always caveats and this method isn't without negatives. The one I could think of was that I cannot monitor the progress of the seed like I can with tissue or cotton pads. Apart from that, I couldn't think of any other downsides to this method. If you think of any, pop em in the post below and I'll add them here. I got the idea from watching several videos and reading about how the commercial guys do things. Check out this video below that kind of explains how they do it if you're planting 100,000s a season. Its packed with information like how they store their seeds.
I've also implemented a peroxide soak based on information provided by Debacco University. I've also linked the video below. This step isn't required but not only does it speed up the process considerably, It ensures that your seed has the best chance to start no matter how old or abused your seed is :D You could also take it one step further and add some sucrose to the water while soaking if your seeds are like 2 million years old or something.
It's probably important to say this method won't resurrect shit seeds lol. Sometimes the seeds are just a lost cause. You could try more advanced techniques like adding sucrose or gibberellic acid but I'd only go that far if the seeds were super rare or valuable.
Required equipment:
I've broken things down into three sections; essentials, beneficial and Soilless seedling mix. Essentials are required to carry out this method. Beneficial are optional but will enhance the plants once germinated as the microlife can get to work as soon as that radical has been established. Soilless seedling mix is required if you would like to use my soilless mix.
Essentials:
- Seedling tray * I'm using the root-it trays in this example. I like em because they're nice and deep. You can get them from most reputable grow shops.
- Base media * You can use my seedling mix below or use any seedling mix of your choice. The main points are that there is little to 0 feed included and the media is nice and light.
- Propagator * You could also use plastic bags if you don't have a propagator. We need to get the humidity up to 100% while the seed is germinating.
- Hydrogen Peroxide * I use Growth Technology Liquid Oxygen 11.9% but I will provide an equation that you can use to achieve the 1% dilution rate no matter what the concentration.
- Vermiculite
- Cup to soak seeds
- Measuring jug
- Spray bottle
- Mycorrhizal * I use TNC MycorrHydro but you can use anything you like. If you're using a powder, mix the recommended dose into your base media.
- Trichoderma I use TNC BactorrS13 within this example but you could use any source. If you're using a powder, mix the recommended dose into your base media.
- Peat * I use Plagron ProMix within this example but you can use any peat you like.
- Perlite
- Mixing tray
Soilless seed mix:
If you're using another base media you can skip this section.
Seeds don't need any nutrients to start. If anything, feeding them too much can cause more issues than good. For this reason, it's best to start with a seedling mix that has a minimal amount of food. After a little research, I've come up with the following seedling mix recipe that seems to work the best for cannabis seeds. Cannabis seems to like light, airy media so I have created this to be a very light mix with great air to water ratio.
Mix 1ltr of peat with 200ml of Perlite and 100ml of Vermiculite. If you're adding the beneficial extras, add them too. Once finished, your media should look like below. Your media is now ready for use.


Soak the seeds:
We're going to be soaking our seeds in a 1% Hydrogen Peroxide solution as outlined in the video provided above. To make a 1% solution, we can use the following equation to determine how much peroxide to add to our water so we have a 1% solution. Soaking the seeds in the 1% peroxide dilute will sterilize the husk, soften the hard shell and allow the seed to take on water and oxygen faster than it would without using it. This in turn speeds up the germination rate as well as improves germination percentages when germinating old seed.
total desired amount of solution / peroxide concentration percentage
For example, say I wanted to make up 100ml of 1% solution and my peroxide was 3%. I can use the above to calculate how much solute I need to add to the solution to achieve 1%
100 (ml) / 3 = 33.3ml
So I would need to add ~33ml to 100ml of water to achieve 1%. Lets try another, we can do the same using GT Liquid Oxygen. We know the concentration percentage is 11.9% so
100 (ml) / 11.9 = 8.4ml
So I would need 8.4ml of GT Liquid Oxygen to achieve the 1% dilution rate. Scary numbers if you go by the manufactures recommended dose but trust the process.
Once you've mixed up your peroxide dilute, add some to your cup and drop the seeds in. If you're germinating different phenotype, use more than one cup to keep things separated. You can soak them for a maximum of around 24 hours or till they sink but I only recommend 4-5 hours unless you have a really good reason to soak them for longer. Maybe if they were really old seeds I'd soak them for 18-24 hours.4-5 hours is more than sufficient for most seeds. Don't worry if they're still floating, that method of determining viability is bullshit anyway
Place the cup in a warm, dark area like a draw of the airing cupboard and let em soak. Since they're going to be soaking for a while, let's go fill the trays with media.


Prepare the seedling trays:
First, take the tray and cut out how many cells you need. Next, fill the cells with your media of choice up to the top of each cell. Next, use your thumb to push back the media so there's about a 2.5mm gap from the top of the cell. From tinkering and other information available online, I've found that the optimal amount of topper is 2.5mm when using Vermiculite. Please use the image below for reference.

Now we're ready to plant the seeds.
Planting the seeds:
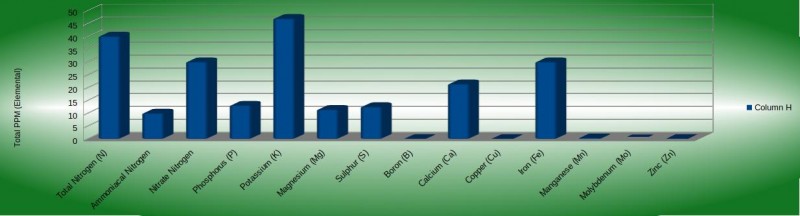
You've soaked the seeds and your seedling tray has been prepared. Now you're ready to sow the seeds and tuck them away for germination. Before we start, we will need to make up the solution that we're going to use to wet the media down after planting the seeds. You can use any fertilizer you like but this should be used sparingly. You could also use a starting feed like Canna Start or Formulex but we don't need much. I'm raising the EC to about 0.6 within this example. I'm using Terra Aquatica Tri-Part at a 0.5/0.5/0.5 ml/L mix with a smidge of Nitrozyme and then adjusting the PH using a little Potassium silicate. See the chart below for a breakdown of each element. The chart below doesn't factor in the silicone but that's only a minimal amount to raise the PH slightly. The PH should be ~6.2PH.
Fill up your spray bottle with the solution and we can move onto the next section.


First, let's spray the cells down to add some moisture. We're not trying to saturate the media but to just wet things lightly. If you're using a manual trigger sprayer, I'd recommend around 12 pumps per section if that makes sense. If you're using a pressure sprayer, then spray each section for around 2-3 seconds. The PH of the media should now be ~6.2PH.

Next, make a little dimple in the middle of the media to hold the seed. I tend to use a pen to made the dimple. It doesn't need to be more than 1mm deep. Next, drop your seeds into the seedling tray so they sit in the dimple that you made previously.

Now top the cells with Vermiculite and knock off any excess. Then water the top of the Vermiculite using the same measurement we used previously. Vermiculite has the perfect water retention capabilities for germination as well as being light as well as having excellent CEC properties. Use the image below for reference.

Finally, place the seedling tray/s into the propagator and switch your lights on. You want to be giving them around 100-150ppfd for now but you can go higher if you wanted to. The important thing is to keep things consistent. Keep the lights on for 24/7 to create the most consistent environment within your propagator. You should aim for a temperature of around 23C within the propagator. You don't want to exceed 26C and at 30C you can wave bye to those seedlings. Monitor the seeds daily for signs of activity. If you did everything correct, everything should be above ground within seven days.




